Malaria vaccine news 2015: World's first malaria vaccine gets 'Green Light' from European drug regulators
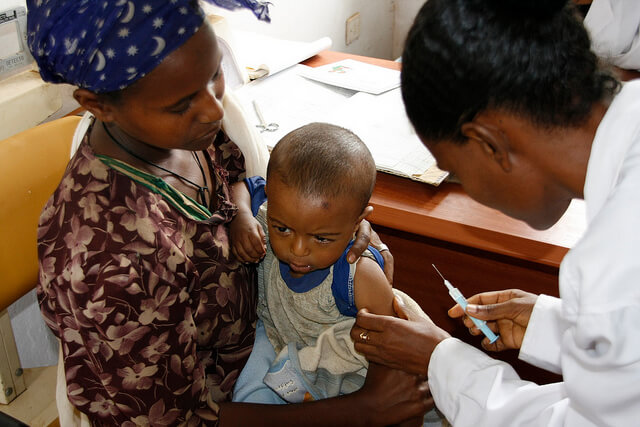
European drug regulators have just given their approval for the world's first ever vaccine for malaria last Thursday.
The European Medicines Agency (EMA) gave a positive feedback on the formulation after it has been proven to be safe and effective in babies.
BBC reported that the vaccine, called RTS,S (Mosquirix) and developed by GlaxoSmithKline (GSK) in partnership with PATH Malaria Vaccine Initiative, will be the first licensed vaccine for malaria that's intended for human use, which could help prevent cases of the disease in commonly affected regions such as Africa.
Other vaccines are designed for the fight against certain viruses or bacteria, but RTS,S was made to target the Plasmodium falciparum parasite that prevalently causes malaria in the sub-Saharan Africa, said Glaxo in a statement based on the report from NBC News.
The parasite that causes malaria is carried by mosquitoes and they can live for years inside the human body.
Usually, vaccines that target parasites are harder to make since these organisms have a complex life cycle.
The vaccine elicits an immune response so more antibodies will be produced to stop the disease from affecting the liver.
It was also noted that the formulation is intended for use in children only or those who are six months to 17 months old, and not for adults or travelers.
RTS,S may also not work effectively on its own, said GSK executive Andrew Witty, according to ABC Online.
Common interventions such as mosquito nets and insect sprays would still help curb malaria cases in African communities, he said.
Despite the fact that the vaccine only works in very young children, EMA recommends it still gets licensed for use in babies. Some scientists may worry about that the not-so effective vaccines may have more pros than cons.
Nevertheless, Africa is still in dire need for such vaccine, even if it's not 100 percent effective.
With the approval coming from EMA , the World Health Organization will step in and decide later on this year if the vaccine should be recommended for use.











